Indications: Nerlynx® (neratinib) tablets, for oral use, is a kinase inhibitor indicated:
- As a single agent, for the extended adjuvant treatment of adult patients with early-stage HER2-positive breast cancer, to follow adjuvant trastuzumab-based therapy.
Reduce the risk of recurrence in early-stage HER2+ breast cancer1,2
Reduction in recurrence consistently observed in the ITT population and across select subgroups1,3
PRIMARY ENDPOINT: ITT population (N=2840)3,*
34% reduction in risk of recurrence at 2 years
94.2% with NERLYNX (n=1420; 95% CI: 92.6%-95.4%) vs 91.9% with placebo (n=1420; 95% CI: 90.2%-93.2%) (HR=0.66; 95% CI: 0.49-0.90; P=0.008)
DESCRIPTIVE ANALYSES: HER2+ HR+ ≤1 year post-trastuzumab subgroup (n=1334)1,*,†
42% reduction in risk of recurrence at 5 years
90.8% with NERLYNX (n=670) vs 85.7% with placebo (n=664) (HR=0.58; 95% CI: 0.41-0.82)
no-pCR subgroup (n=295)1,*,†
40% reduction in risk of recurrence at 5 years
85.0% with NERLYNX (n=131) vs 77.6% with placebo (n=164) (HR=0.60; 95% CI: 0.33-1.07)
HR+ ≤1 year post-trastuzumab and no-pCR subgroups were descriptive analyses, not prespecified or powered.1
- “Recurrence” was defined as time from randomization to first occurrence of invasive ipsilateral tumor recurrence, invasive contralateral breast cancer, local/regional invasive recurrence, distant recurrence, or death from any cause.1
- Results of ExteNET are supported by descriptive analyses after 5 years of follow-up, with 75% of patients (2117/2840) reconsented. 95% of the patients with HER2+ HR+ disease had concomitant endocrine therapy.1,2
- CI: confidence interval; HR: hazard ratio; HR+: hormone receptor–positive; iDFS: invasive disease–free survival; ITT: intent to treat; pCR: pathologic complete response.
Select Important Safety Information
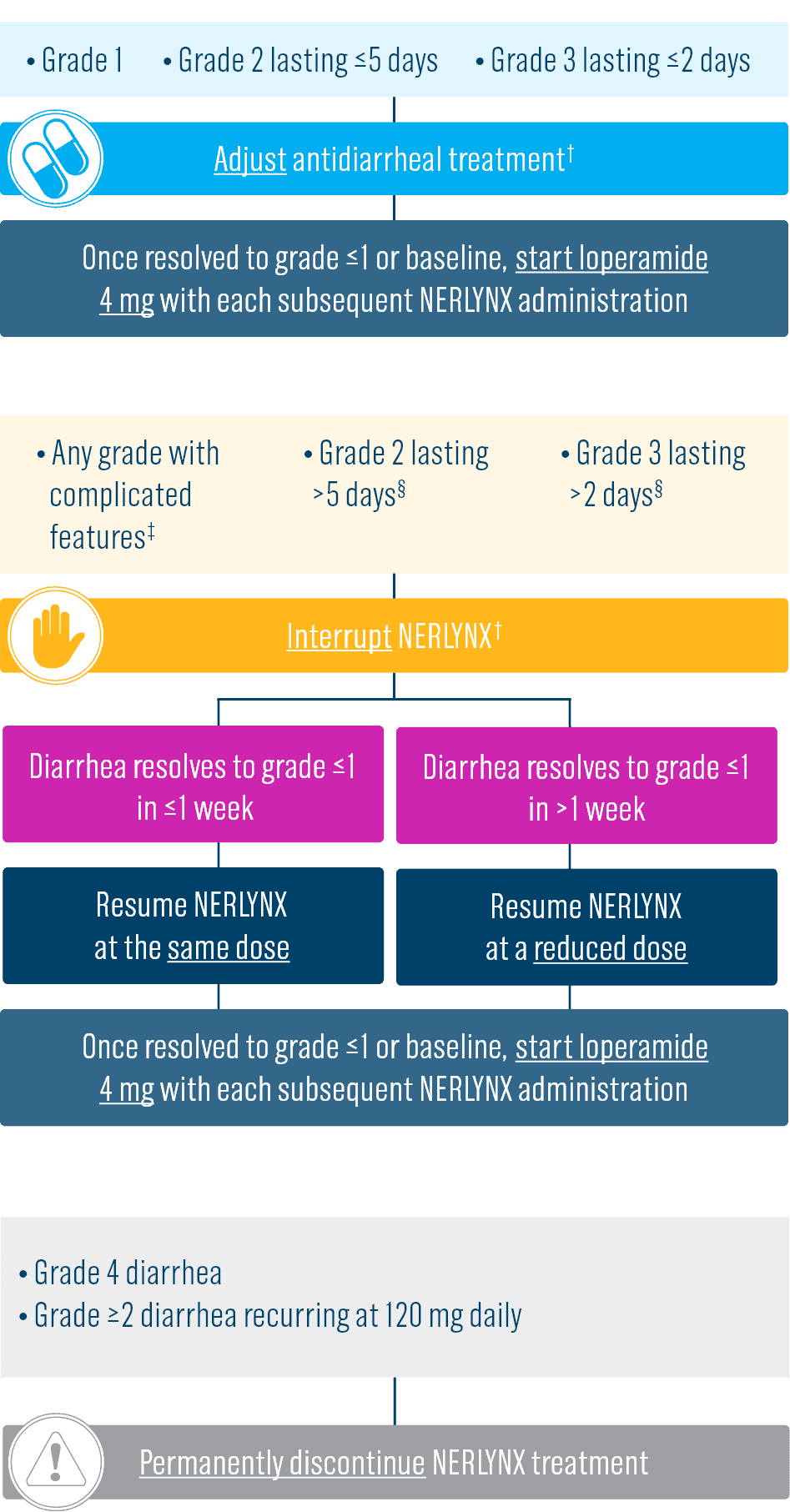
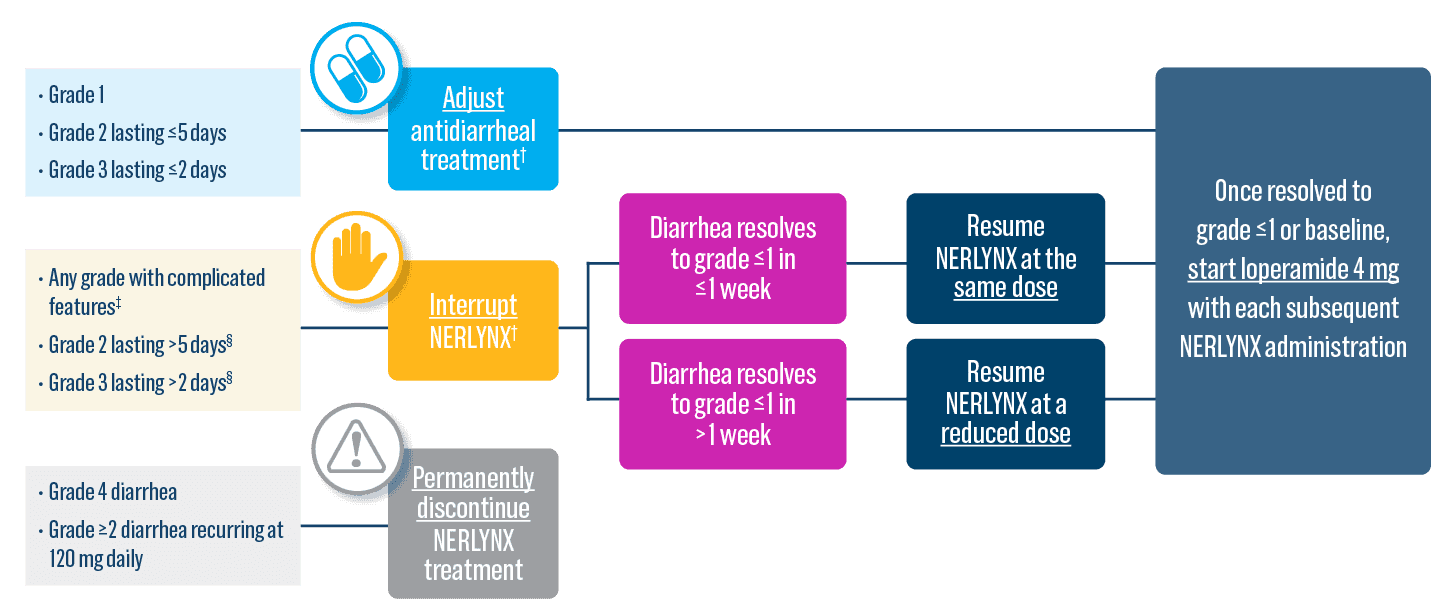
- Diarrhea: Manage diarrhea through either Nerlynx dose escalation or loperamide prophylaxis. If diarrhea occurs despite recommended prophylaxis, treat with additional antidiarrheals, fluids, and electrolytes as clinically indicated. Withhold Nerlynx in patients experiencing severe and/or persistent diarrhea. Permanently discontinue Nerlynx in patients experiencing Grade 4 diarrhea or Grade ≥2 diarrhea that occurs after maximal dose reduction.
Please see additional Important Safety Information below.
Observed iDFS impact across several prespecified subgroups4
ITT population: iDFS benefit at 2 years, by subgroup4
Subgroup analyses were prespecified but no adjustment was made for multiple comparisons.
- CI: confidence interval; HR: hazard ratio; iDFS: invasive disease–free survival; ITT: intent to treat.
Overall survival observed in select subgroups1
DESCRIPTIVE ANALYSES: HER2+ HR+ ≤1 year post-trastuzumab subgroup (n=1334)1,*
21% reduction in risk of death at a median follow-up of 8 years
91.5% with NERLYNX (n=670) vs 89.4% with placebo (n=664) (HR=0.79; 95% CI: 0.55-1.13)
NO-pCR subgroup (n=295)1,*
53% reduction in risk of death at a median follow-up of 8 years
91.3% with NERLYNX (n=131) vs 82.2% with placebo (n=164) (HR=0.47; 95% CI: 0.23-0.92)
Descriptive analyses, not prespecified or powered.1
After a median follow-up of 8 years, there was no statistically significant difference in OS between the Nerlynx arm and the placebo arm in the ITT population (HR=0.95; 95% CI: 0.75-1.21).3
- Results of ExteNET are supported by descriptive analyses after 5 years of follow-up, with 75% of patients (2117/2840) reconsented. 95% of the patients with HER2+ HR+ disease had concomitant endocrine therapy.1,2
- CI: confidence interval; HR: hazard ratio; HR+: hormone receptor–positive; ITT: intent to treat; OS: overall survival; pCR: pathologic complete response.
Select Important Safety Information
- Hepatotoxicity: Monitor liver function tests monthly for the first 3 months of treatment, then every 3 months while on treatment and as clinically indicated. Withhold Nerlynx in patients experiencing Grade 3 liver abnormalities and permanently discontinue Nerlynx in patients experiencing Grade 4 liver abnormalities.
Please see additional Important Safety Information below.
CNS recurrence as the first site of breast cancer metastases observed in select subgroups1
DESCRIPTIVE ANALYSES: HER2+ HR+ ≤1 year post-trastuzumab subgroup (n=1334)1,*
1.4% absolute reduction in cumulative incidence of CNS recurrences as the first site of metastases at 5 years
0.7% of patients (n=670; 95% CI: 0.2%-1.7%) experienced CNS recurrence as the first site of metastases with NERLYNX vs 2.1% of patients (n=664; 95% CI: 1.1%-3.5%) with placebo
NO-pCR subgroup (n=295)1,*
2.8% absolute reduction in cumulative incidence of CNS recurrences as the first site of metastases at 5 years
0.8% of patients (n=131; 95% CI: 0.1%-4.0%) experienced CNS recurrence as the first site of metastases with NERLYNX vs 3.6% of patients (n=164; 95% CI: 1.3%-7.8%) with placebo
Descriptive analyses, not prespecified or powered; disease recurrence beyond first event may not have been consistently collected for meaningful statistical analysis.1
- Results of ExteNET are supported by descriptive analyses after 5 years of follow-up, with 75% of patients (2117/2840) reconsented. 95% of the patients with HER2+ HR+ disease had concomitant endocrine therapy.1,2
- CI: confidence interval; CNS: central nervous system; HR+: hormone receptor–positive; pCR: pathologic complete response.
Impact on risk of CNS recurrence in select subgroups1
DESCRIPTIVE ANALYSES: HER2+ HR+ ≤1 year post-trastuzumab subgroup (n=1334)1,*
59% reduction in the risk of CNS recurrence or death from any cause at 5 years
98.4% of patients (n=670; 95% CI: 96.8%-99.1%) experienced CNS-DFS at 5 years with NERLYNX vs 95.7% of patients (n=664; 95% CI: 93.6%-97.2%) with placebo (HR=0.41; 95% CI: 0.18-0.85)
NO-pCR subgroup (n=295)1,*
76% reduction in the risk of CNS recurrence or death from any cause at 5 years
98.4% with NERLYNX (n=131; 95% CI: 93.6%-99.6%) vs 92.0% with placebo (n=164; 95% CI: 85.6%-95.7%) (HR=0.24; 95% CI: 0.04-0.92)
Descriptive analyses, not prespecified or powered; disease recurrence beyond first event may not have been consistently collected for meaningful statistical analysis.1
- Results of ExteNET are supported by descriptive analyses after 5 years of follow-up, with 75% of patients (2117/2840) reconsented. 95% of the patients with HER2+ HR+ disease had concomitant endocrine therapy.1,2
- CI: confidence interval; CNS: central nervous system; DFS: disease-free survival; HR: hazard ratio; HR+: hormone receptor–positive; pCR: pathologic complete response.
Nerlynx safety in ExteNET3
Most common adverse reactions (≥5%)
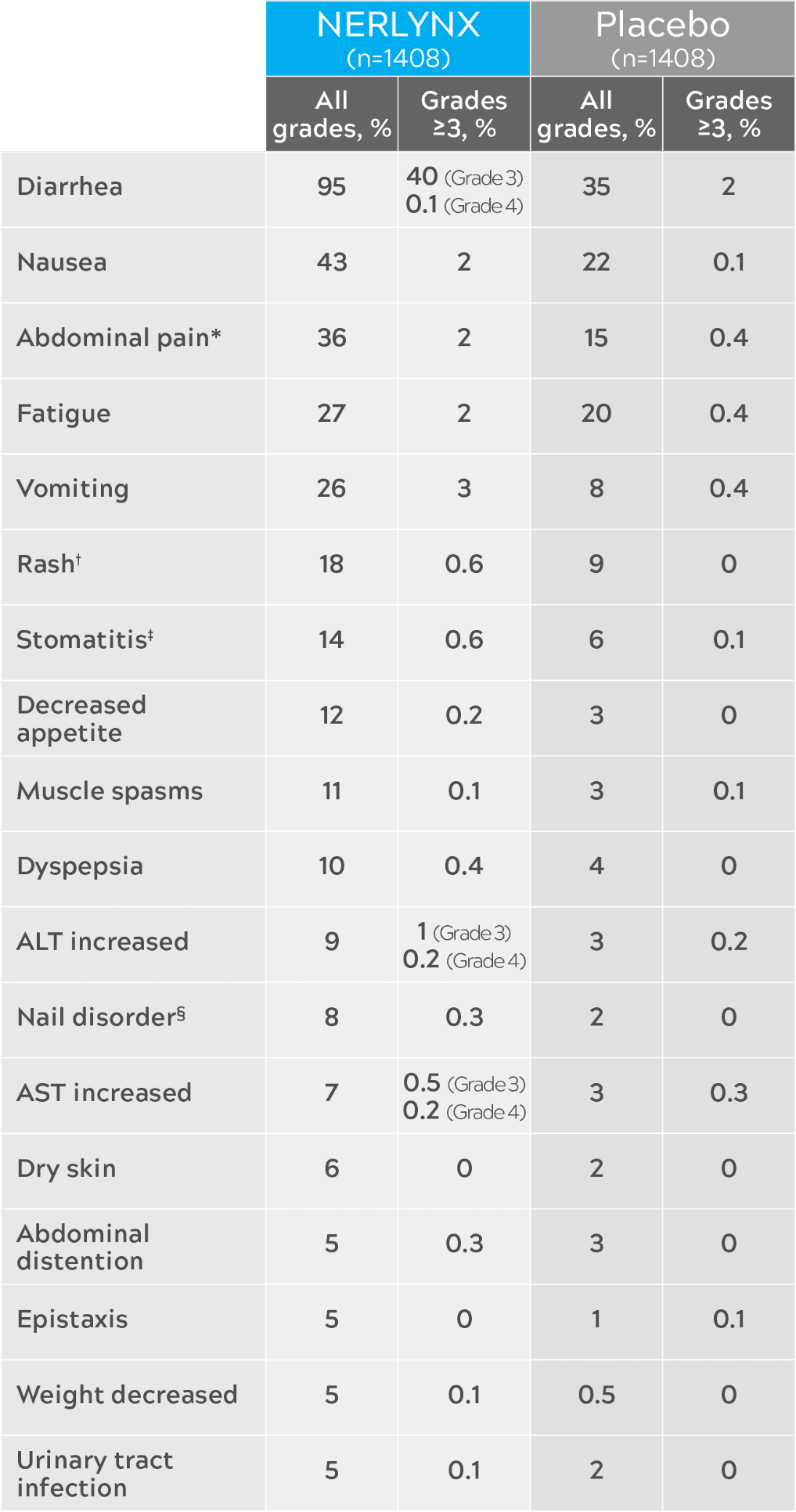
| Nerlynx (n=1408) | Placebo (n=1408) | |||
|---|---|---|---|---|
| All grades, % | Grades ≥3, % | All grades, % | Grades ≥3, % | |
| Diarrhea | 95 | 40 (Grade 3) 0.1 (Grade 4) | 35 | 2 |
| Nausea | 43 | 2 | 22 | 0.1 |
| Abdominal pain* | 36 | 2 | 15 | 0.4 |
| Fatigue | 27 | 2 | 20 | 0.4 |
| Vomiting | 26 | 3 | 8 | 0.4 |
| Rash† | 18 | 0.6 | 9 | 0 |
| Stomatitis‡ | 14 | 0.6 | 6 | 0.1 |
| Decreased appetite | 12 | 0.2 | 3 | 0 |
| Muscle spasms | 11 | 0.1 | 3 | 0.1 |
| Dyspepsia | 10 | 0.4 | 4 | 0 |
| ALT increased | 9 | 1 (Grade 3) 0.2 (Grade 4) | 3 | 0.2 |
| Nail disorder§ | 8 | 0.3 | 2 | 0 |
| AST increased | 7 | 0.5 (Grade 3) 0.2 (Grade 4) | 3 | 0.3 |
| Dry skin | 6 | 0 | 2 | 0 |
| Abdominal distention | 5 | 0.3 | 3 | 0 |
| Epistaxis | 5 | 0 | 1 | 0.1 |
| Weight decreased | 5 | 0.1 | 0.5 | 0 |
| Urinary tract infection | 5 | 0.1 | 2 | 0 |
The USPI warnings and precautions do not include cardiac, pulmonary, or hematologic toxicities, or increased risk for secondary malignancy.3
Drug Interactions3
- Gastric acid–reducing agents: Avoid concomitant use with proton pump inhibitors. Separate Nerlynx by at least 2 hours before or 10 hours after H2-receptor antagonists. Or separate Nerlynx by at least 3 hours after antacids
- Strong CYP3A4 inhibitors: Avoid concomitant use
- P-gp and moderate CYP3A4 dual inhibitors: Avoid concomitant use
- Strong or moderate CYP3A4 inducers: Avoid concomitant use
- Certain P-gp substrates: Monitor for adverse reactions of P-gp substrates for which minimal concentration change may lead to serious adverse reactions when used concomitantly with Nerlynx
- Includes abdominal pain, abdominal pain upper, and abdominal pain lower.3
- Includes rash, rash erythematous, rash follicular, rash generalized, rash pruritic, rash pustular, rash maculo-papular, rash papular, dermatitis, dermatitis acneiform, and toxic skin eruption.3
- Includes stomatitis, aphthous stomatitis, mouth ulceration, oral mucosal blistering, mucosal inflammation, oropharyngeal pain, oral pain, glossodynia, glossitis, and cheilitis.3
- Includes nail disorder, paronychia, onychoclasis, nail discoloration, nail toxicity, nail growth abnormal, and nail dystrophy.3
- ALT: alanine aminotransferase; AST: aspartate aminotransferase.
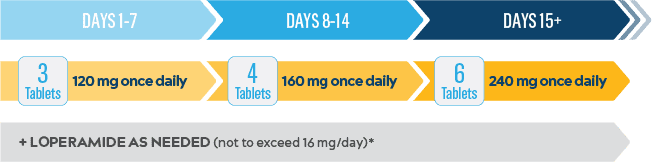
Start Nerlynx at a lower dose and titrate up to the full recommended dose to help manage diarrhea3
Nerlynx Dose Escalation3
13% rate of grade 3 diarrhea with Nerlynx dose escalation3,†
- Compared to 40% with Nerlynx in ExteNET, the dose-escalation arm in CONTROL had a >65% lower rate of grade 3 diarrhea
2.5 median cumulative days of grade ≥3 diarrhea with NERLYNX dose escalation3,†
- Compared to 5 days with Nerlynx in ExteNET, the dose-escalation arm in Control had 50% fewer median days of grade ≥3 diarrhea
3.3% treatment discontinuations due to diarrhea with Nerlynx dose escalation3,†
- Compared to 17% with Nerlynx in ExteNET, the dose-escalation arm in CONTROL had a >80% lower rate of diarrhea-related treatment discontinuations
Loperamide-prophylaxis arm of Control: 32% grade 3 diarrhea (n=109),3 3-day median cumulative duration of grade ≥3 diarrhea (n=137),6 and 18% discontinuation rate due to diarrhea (n=109).3

Loperamide-prophylaxis arm of Control: 32% grade 3 diarrhea (n=109),3 3-day median cumulative duration of grade ≥3 diarrhea (n=137),6 and 18% discontinuation rate due to diarrhea (n=109).3
Control was a phase 2, open-label, multicohort, multinational study that evaluated the effect of dose escalation or antidiarrheal prophylaxis on diarrhea associated with Nerlynx. Nerlynx dose-escalation arm DE1†: n=60; 120 mg/day on days 1-7, 160 mg/day on days 8-14, 240 mg/day on days 15-364.6
ExteNET was a pivotal phase 3, global, multicenter, randomized, double-blind, placebo-controlled study. Nerlynx arm in ExteNET: n=1408; 240 mg/day for up to 1 year. Antidiarrheals were not protocol mandated.1,3
Control was a phase 2, open-label, multicohort, multinational study that evaluated the effect of dose escalation or antidiarrheal prophylaxis on diarrhea associated with Nerlynx. Nerlynx dose-escalation arm DE1†: n=60; 120 mg/day on days 1-7, 160 mg/day on days 8-14, 240 mg/day on days 15-364.6
ExteNET was a pivotal phase 3, global, multicenter, randomized, double-blind, placebo-controlled study. Nerlynx arm in ExteNET: n=1408; 240 mg/day for up to 1 year. Antidiarrheals were not protocol mandated.1,3
- If diarrhea occurs, treat with antidiarrheal medications, fluids, and electrolytes as clinically indicated.3
- Data from Nerlynx dose-escalation arm DE1 in Control. There was an additional Nerlynx dose-escalation arm, DE2, studied in Control. Data from DE2 are not included in the USPI.3,6
- eBC: early-stage breast cancer.
Support for patients prescribed NERLYNX*
- Access and coverage
- Ongoing patient support
Puma Patient Lynx programs are subject to change or to be discontinued without notice. Limitations apply and certain programs are subject to eligibility criteria. For full terms and conditions, call 1-855-816-5421.
Download the Nerlynx Dosing and Administration Guide for additional information to help your patients get started with Nerlynx
Once-daily oral dosing with Nerlynx
Important Dosing Information3
- The recommended dose is 240 mg once daily*
- A 2-week dose escalation for Nerlynx may also be initiated
- Instruct patients to take Nerlynx with food at approximately the same time every day
- Nerlynx tablets should be swallowed whole (not be chewed, crushed, or split prior to swallowing)
- Patients with early-stage HER2+ breast cancer should take NERLYNX until disease recurrence or up to 1 year
- If a patient misses a dose, do not replace a missed dose, and instruct the patient to resume Nerlynx with the next scheduled daily dose
- Dose interruptions and/or dose reductions are recommended based on individual safety and tolerability
- Hepatic impairment: reduce the starting dose to 80 mg in patients with severe hepatic impairment
- Discontinue Nerlynx for patients with adverse reactions that fail to recover to grade 0-1 or baseline, with toxicities that result in a treatment delay >3 weeks, or if unable to tolerate 120 mg daily
- If diarrhea occurs, treat with antidiarrheal medications, fluids, and electrolytes as clinically indicated.3
Dose adjustments to help manage side effects3
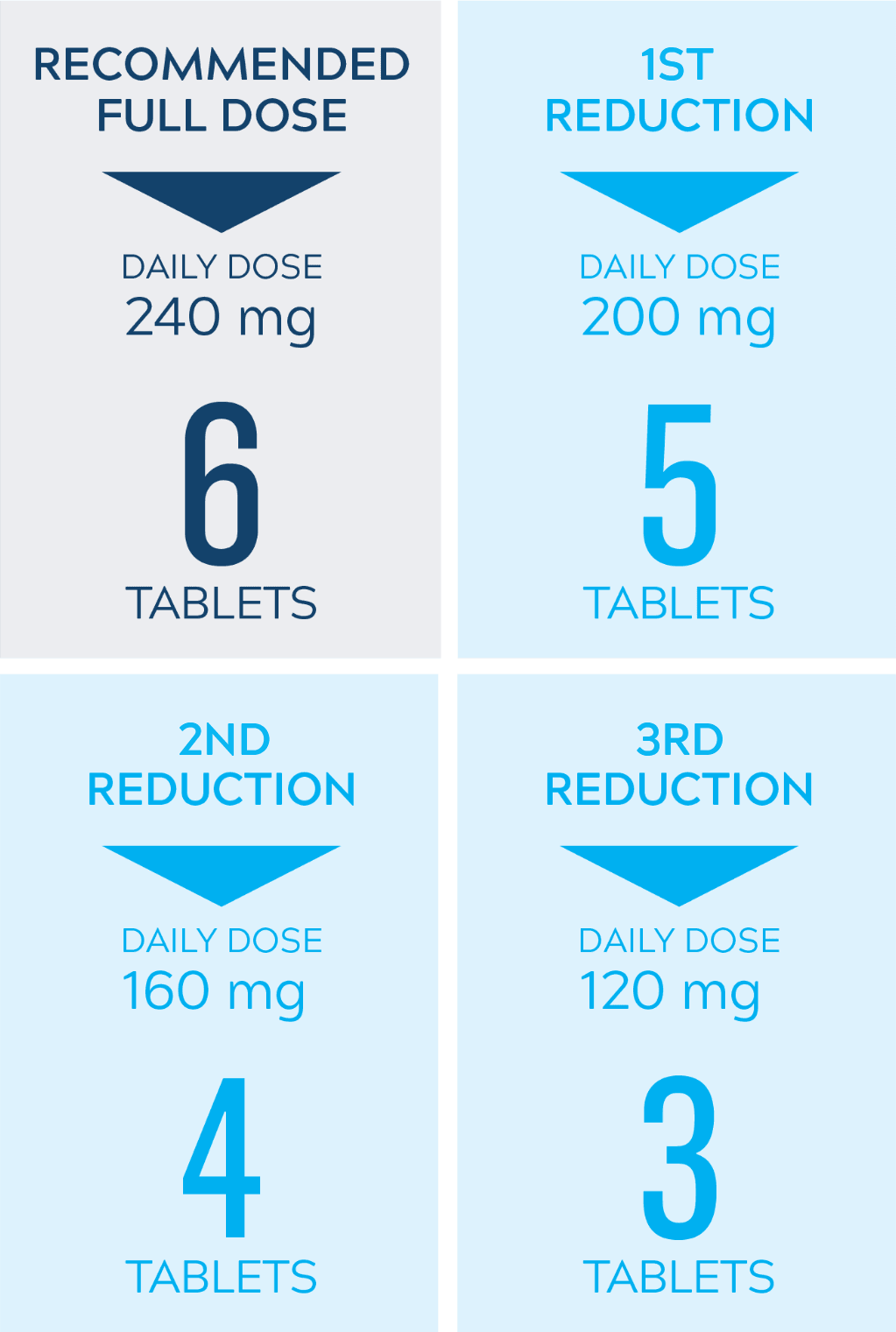
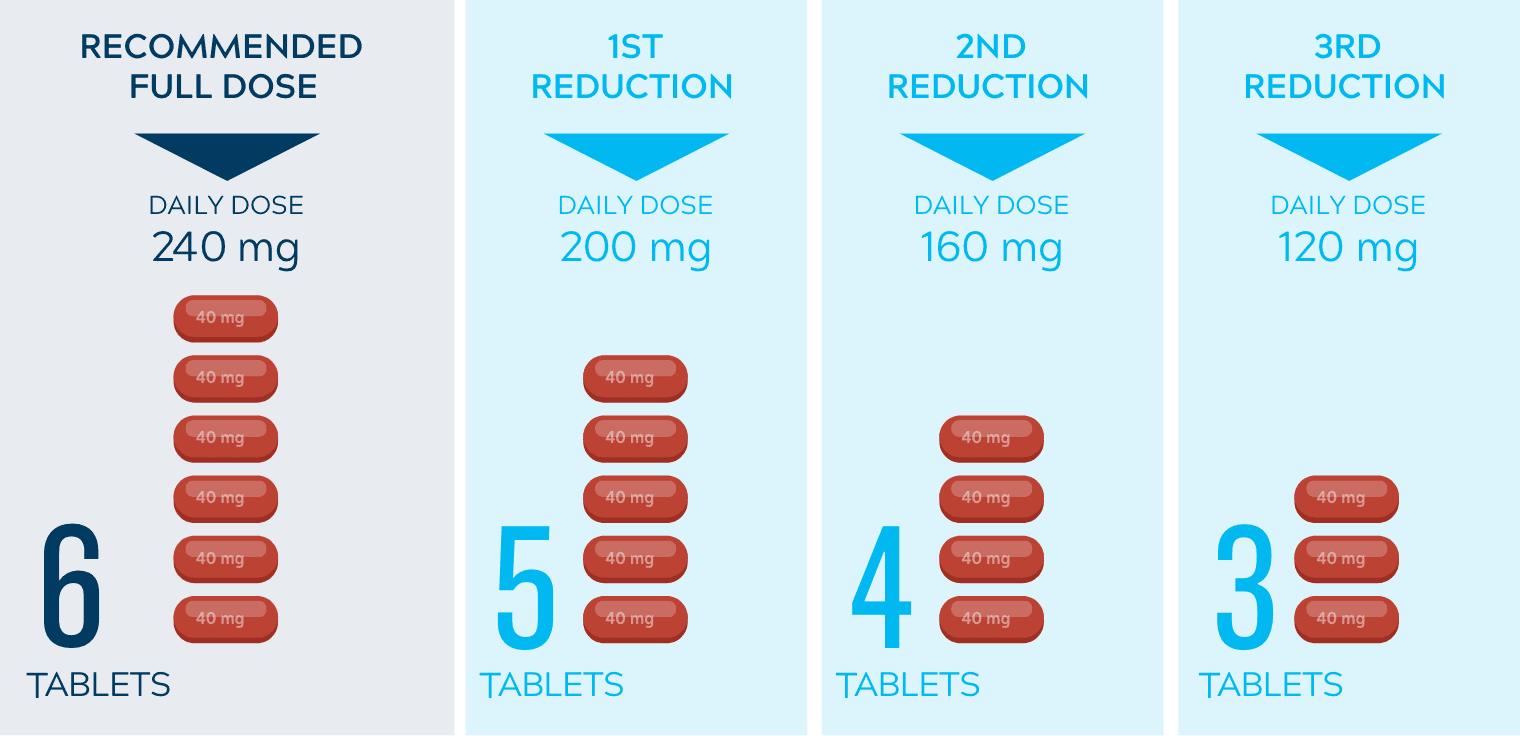

- Nerlynx dose modifications are recommended based on individual safety and tolerability; adjust the dose as clinically indicated
- Some adverse reactions may require dose interruption, reduction, or discontinuation
- Discontinue Nerlynx for patients who fail to recover to grade ≤1 or baseline from treatment-related toxicity, for toxicities that result in a treatment delay >3 weeks, for patients who are unable to tolerate 120 mg daily, or for any grade 4 toxicities
References:
- Chan A, Moy B, Mansi J, et al. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the phase III ExteNET trial. Clin Breast Cancer. 2021;21(1):80-91.e7. doi:10.1016/j.clbc.2020.09.014
- Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688-1700. doi:10.1016/S1470-2045(17)30717-9
- Nerlynx [package insert]. Los Angeles, CA: Puma Biotechnology, Inc.
- Puma Biotechnology, Inc. Data on file.
- Moy B, Takahashi M, Ohtani S, et al. Association between treatment duration and overall survival in early-stage HER2+ breast cancer patients receiving extended adjuvant therapy with neratinib in the ExteNET trial. Poster presented at: American Society of Clinical Oncology (ASCO) Annual Meeting; June 4-8, 2021.
- Chan A, Ruiz-Borrego M, Marx G, et al. Final findings from the Control trial: strategies to reduce the incidence and severity of neratinib-associated diarrhea in patients with HER2-positive early-stage breast cancer. Breast. 2023;67:94-101. doi:10.1016/j.breast.2022.12.003
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. November 27, 2017. Accessed May 24, 2022. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
Important Safety Information and Indications
Contraindications: None
Warnings and Precautions:
- Diarrhea: Manage diarrhea through either Nerlynx dose escalation or loperamide prophylaxis. If diarrhea occurs despite recommended prophylaxis, treat with additional antidiarrheals, fluids, and electrolytes as clinically indicated. Withhold Nerlynx in patients experiencing severe and/or persistent diarrhea. Permanently discontinue Nerlynx in patients experiencing Grade 4 diarrhea or Grade ≥2 diarrhea that occurs after maximal dose reduction.
- Hepatotoxicity: Monitor liver function tests monthly for the first 3 months of treatment, then every 3 months while on treatment and as clinically indicated. Withhold Nerlynx in patients experiencing Grade 3 liver abnormalities and permanently discontinue Nerlynx in patients experiencing Grade 4 liver abnormalities.
- Embryo-Fetal Toxicity: Nerlynx can cause fetal harm. Advise patients of potential risk to a fetus and to use effective contraception.
Adverse Reactions: The most common adverse reactions (reported in ≥5% of patients) were:
- Nerlynx as a single agent: diarrhea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, AST or ALT increased, nail disorder, dry skin, abdominal distention, epistaxis, weight decreased, and urinary tract infection.
- Nerlynx in combination with capecitabine: diarrhea, nausea, vomiting, decreased appetite, constipation, fatigue/asthenia, weight decreased, dizziness, back pain, arthralgia, urinary tract infection, upper respiratory tract infection, abdominal distention, renal impairment, and muscle spasms.
To report suspected adverse reactions, contact Puma Biotechnology, Inc. at 1-844-Nerlynx (1-844-637-5969) or FDA at 1-800-332-1088 or www.fda.gov/medwatch.
Drug Interactions:
- Gastric acid reducing agents: Avoid concomitant use with proton pump inhibitors. Separate Nerlynx by at least 2 hours before or 10 hours after H2-receptor antagonists. Or separate Nerlynx by at least 3 hours after antacids.
- Strong CYP3A4 inhibitors: Avoid concomitant use.
- P-gp and moderate CYP3A4 dual inhibitors: Avoid concomitant use.
- Strong or moderate CYP3A4 inducers: Avoid concomitant use.
- Certain P-gp substrates: Monitor for adverse reactions of P-gp substrates for which minimal concentration change may lead to serious adverse reactions when used concomitantly with Nerlynx.
Use In Specific Populations:
- Lactation: Advise women not to breastfeed.
Please see Full Prescribing Information.
Indications: Nerlynx® (neratinib) tablets, for oral use, is a kinase inhibitor indicated:
- As a single agent, for the extended adjuvant treatment of adult patients with early-stage HER2-positive breast cancer, to follow adjuvant trastuzumab-based therapy.
- In combination with capecitabine, for the treatment of adult patients with advanced or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 based regimens in the metastatic setting.