Indications: Nerlynx® (neratinib) tablets, for oral use, is a kinase inhibitor indicated:
- As a single agent, for the extended adjuvant treatment of adult patients with early-stage HER2-positive breast cancer, to follow adjuvant trastuzumab-based therapy.
Assessing the risk of recurrence in patients with early-stage HER2+ breast cancer
“When I found out I was cancer free, for a moment, I felt some kind of relief . . . but the reality is that I can never really stop worrying.”
Real patient with HER2+ HR+ breast cancer who had a pCR. NERLYNX was not commercially available at the time of treatment. Photograph taken April 2013.
DESPITE ADVANCES IN THE TREATMENT OF HER2+ eBC, THE RISK OF RECURRENCE REMAINS
KATHERINE: PHASE 3 STUDY OF T-DM1 (n=743) VS TRASTUZUMAB (n=743) IN PATIENTS WITH HER2+ eBC WITH NO pCR AFTER NEOADJUVANT TREATMENT1

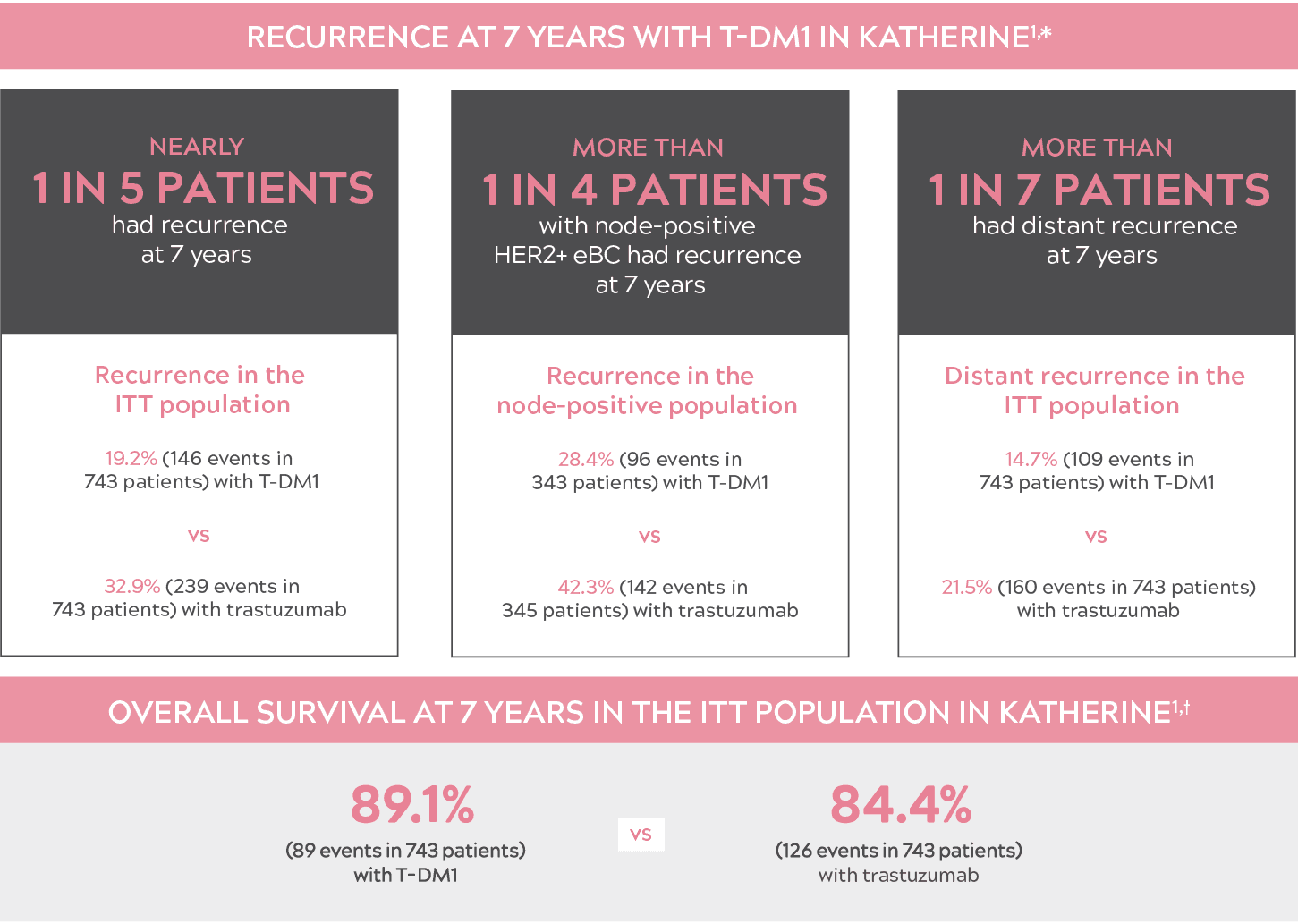

- "Recurrence" was defined as the first occurrence of one of the following events: recurrence of ipsilateral locoregional invasive breast cancer, contralateral invasive breast cancer, a distant disease recurrence, or death from any cause.2
- (HR=0.66; 95% CI: 0.51-0.87; P=0.0027).
- CI: confidence interval; eBC: early-stage breast cancer; HER2+: human epidermal growth factor receptor 2–positive; HR: hazard ratio; ITT: intent to treat; pCR: pathologic complete response; T-DM1: trastuzumab emtansine.
THE RISK OF RECURRENCE IN HER2+ eBC MAY INCREASE OVER TIME
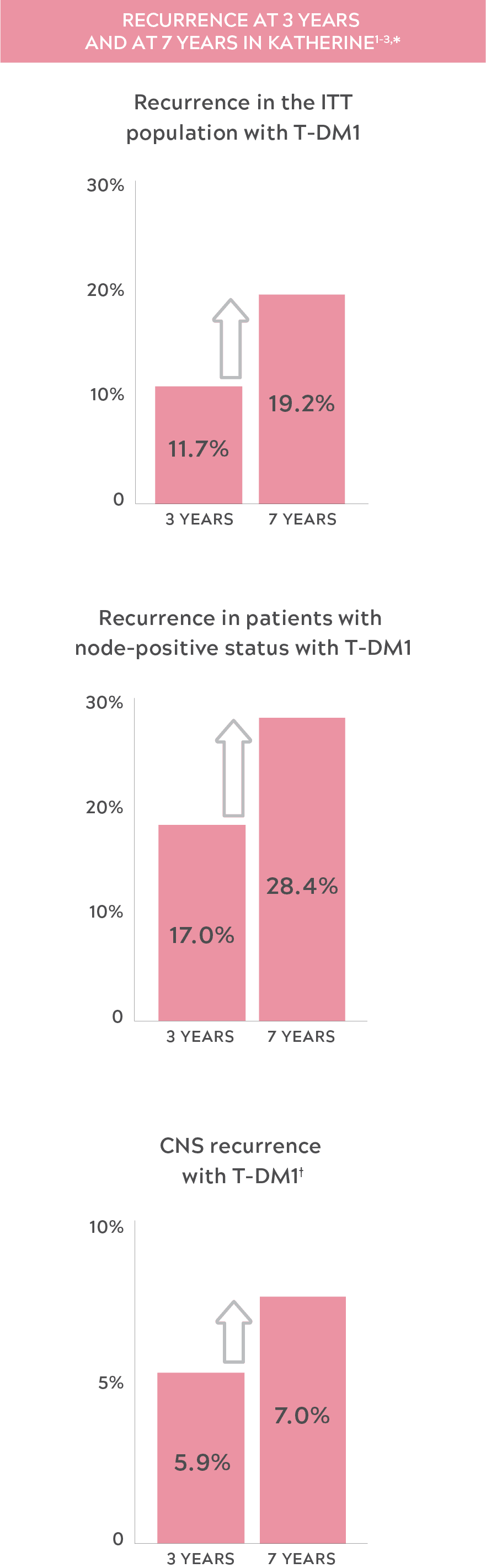
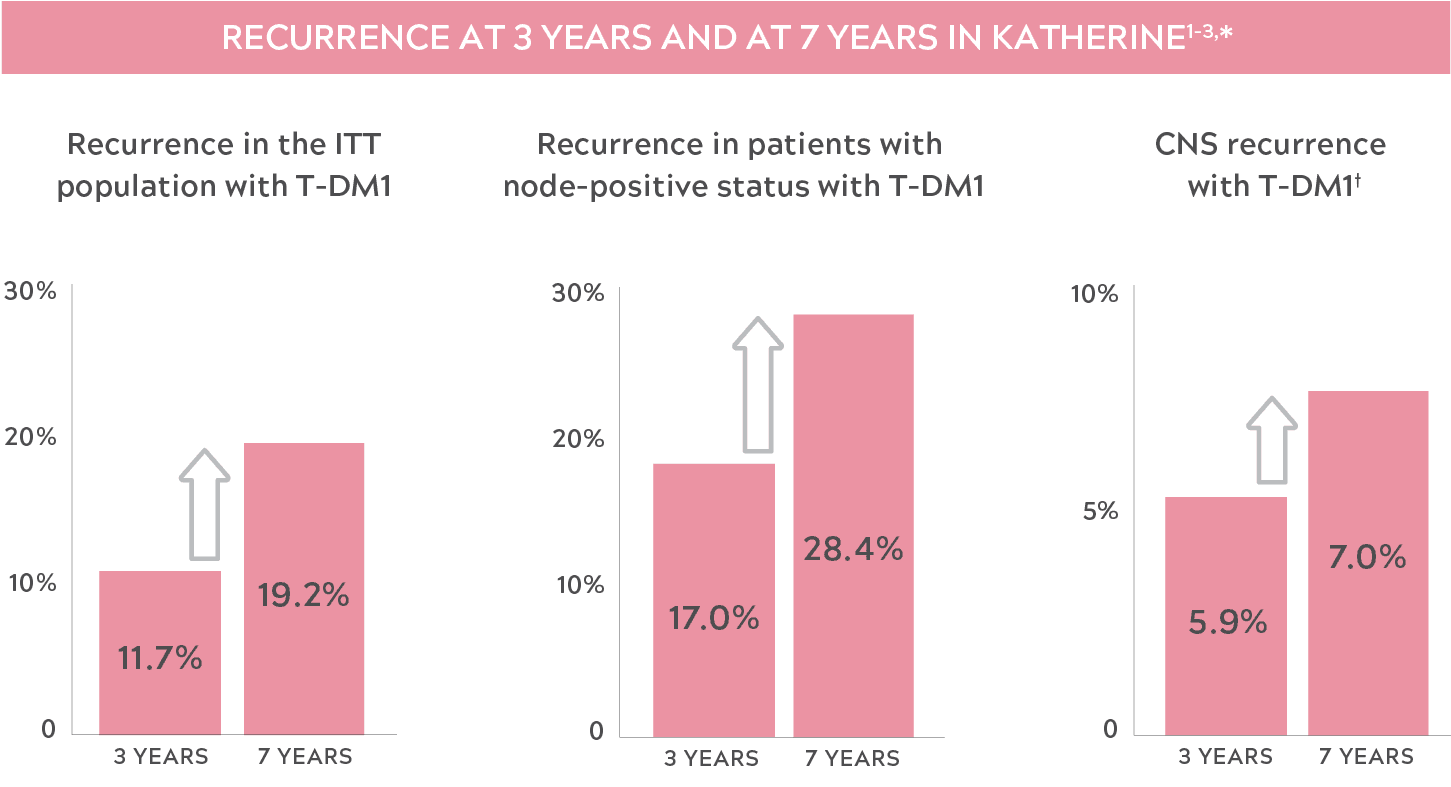

- "Recurrence" was defined as the first occurrence of one of the following events: recurrence of ipsilateral locoregional invasive breast cancer, contralateral invasive breast cancer, a distant disease recurrence, or death from any cause.2
- These data are for CNS recurrence as a site of first occurrence of an iDFS event.2,3
- CNS: central nervous system; eBC: early-stage breast cancer; HER2+: human epidermal growth factor receptor 2–positive; iDFS: invasive disease–free survival; ITT: intent to treat; T-DM1: trastuzumab emtansine.
5-YEAR PFS EVENT RATES IN HER2+ eBC PATIENTS4
EXPLORATORY SUBGROUP ANALYSIS: RESULTS FROM NEOSPHERE4
NEOSPHERE: PHASE 2 STUDY OF NEOADJUVANT CHEMOTHERAPY REGIMENS IN PATIENTS WITH HER2+ eBC4
Pertuzumab + trastuzumab + docetaxel (n=107) vs trastuzumab + docetaxel (n=107) vs pertuzumab + trastuzumab (n=107) vs pertuzumab + docetaxel (n=96)
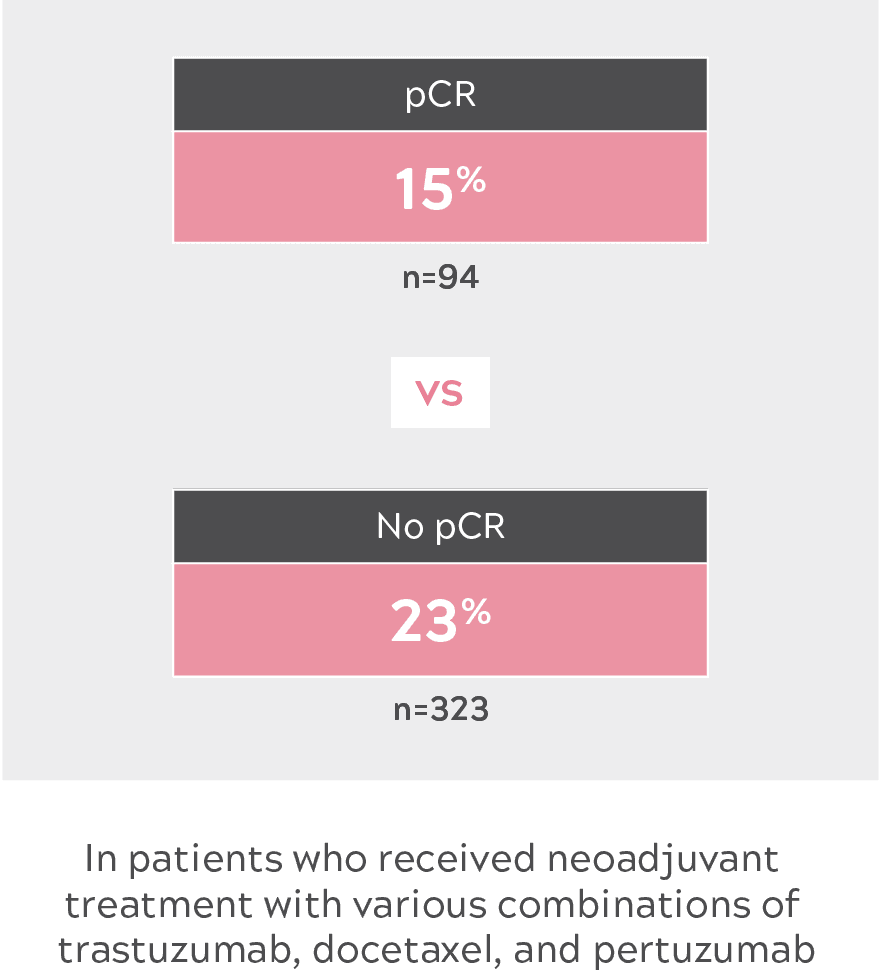


- eBC: early-stage breast cancer; HER2+: human epidermal growth factor receptor 2–positive; pCR: pathologic complete response; PFS: progression-free survival.
THE CNS IS A SANCTUARY SITE FOR HER2+ BREAST CANCER METASTASES5
CNS RECURRENCE RATES IN PATIENTS WHO EXPERIENCED DISTANT RECURRENCE IN HER2+ eBC TRIALS1,6



* KATHERINE: Phase 3 study of T-DM1 (n=743) vs trastuzumab (n=743) in patients with HER2+ eBC with no pCR after neoadjuvant treatment.
† APHINITY: Phase 3 study of pertuzumab (n=2400) vs placebo (n=2404) added to adjuvant chemotherapy followed by trastuzumab in patients with LN+ or high risk LN- HER2+ operable eBC.
- CNS: central nervous system; eBC: early-stage breast cancer; HER2+: human epidermal growth factor receptor 2–positive; LN: lymph node; pCR: pathologic complete response; T-DM1: trastuzumab emtansine.
IMPORTANCE OF ASSESSING THE RISK OF RECURRENCE IN PATIENTS WITH HER2+ eBC
JUST 1 RISK FACTOR AT INITIAL PRESENTATION IS ENOUGH TO INCREASE THE RISK OF RECURRENCE7-11
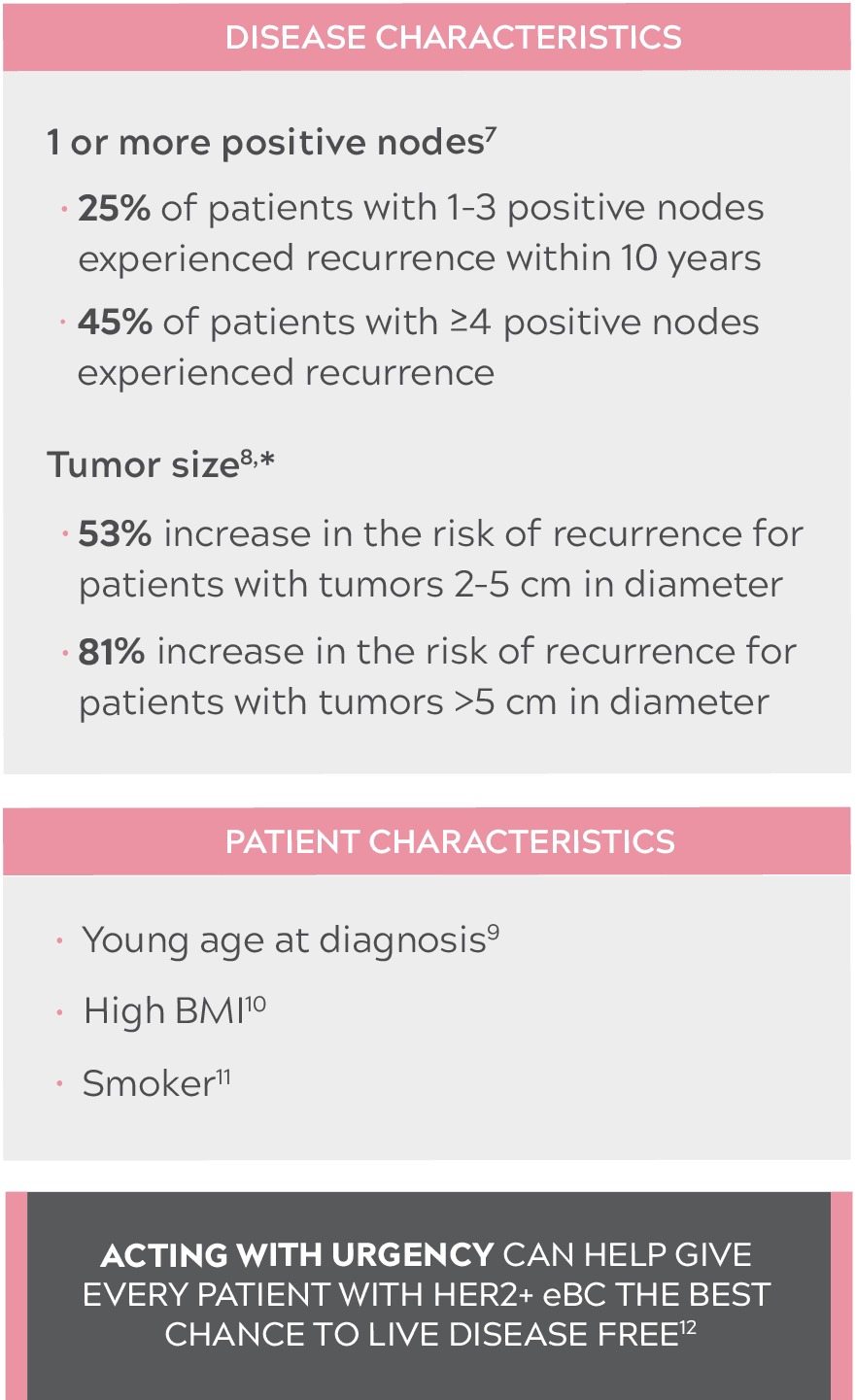
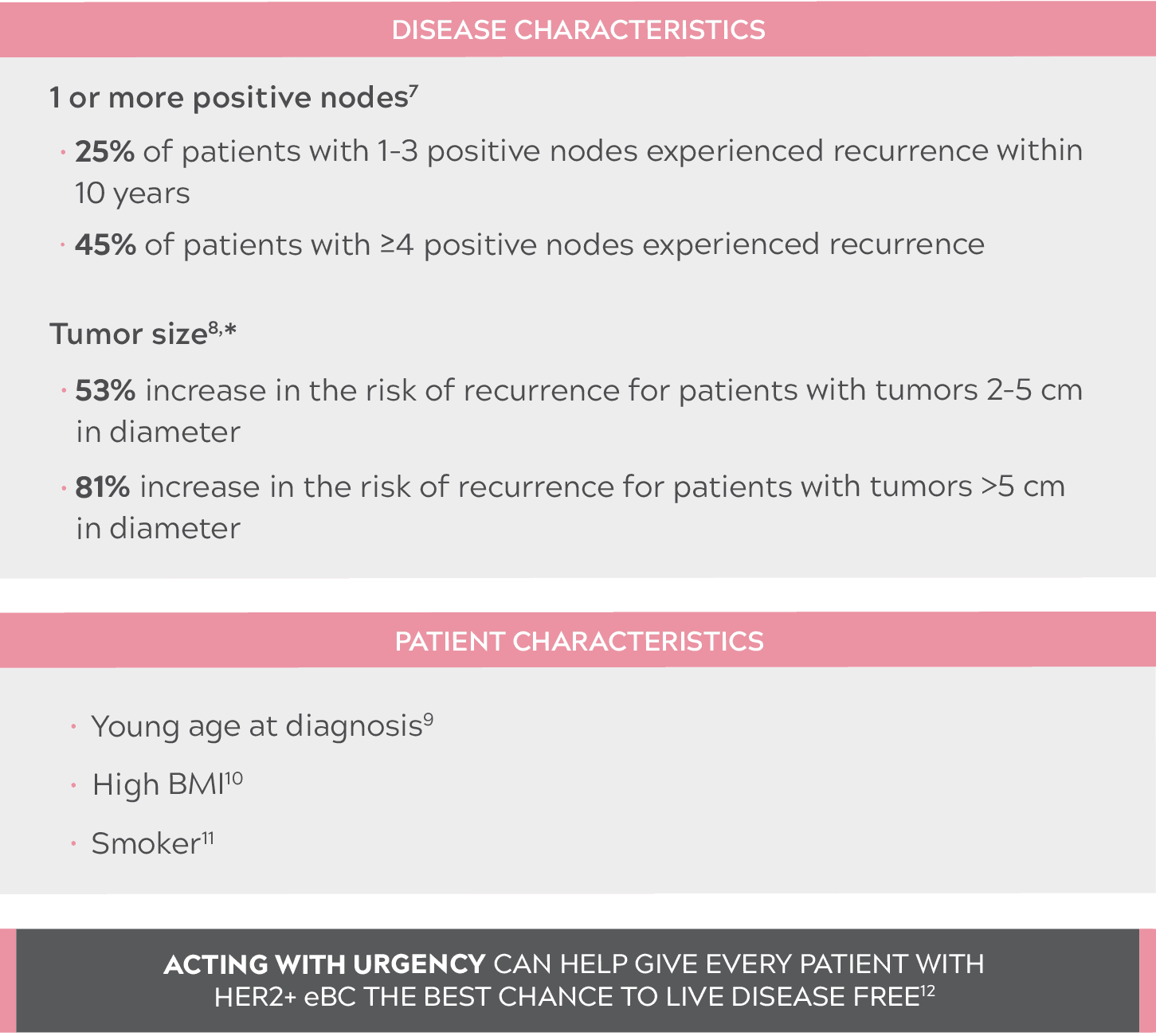
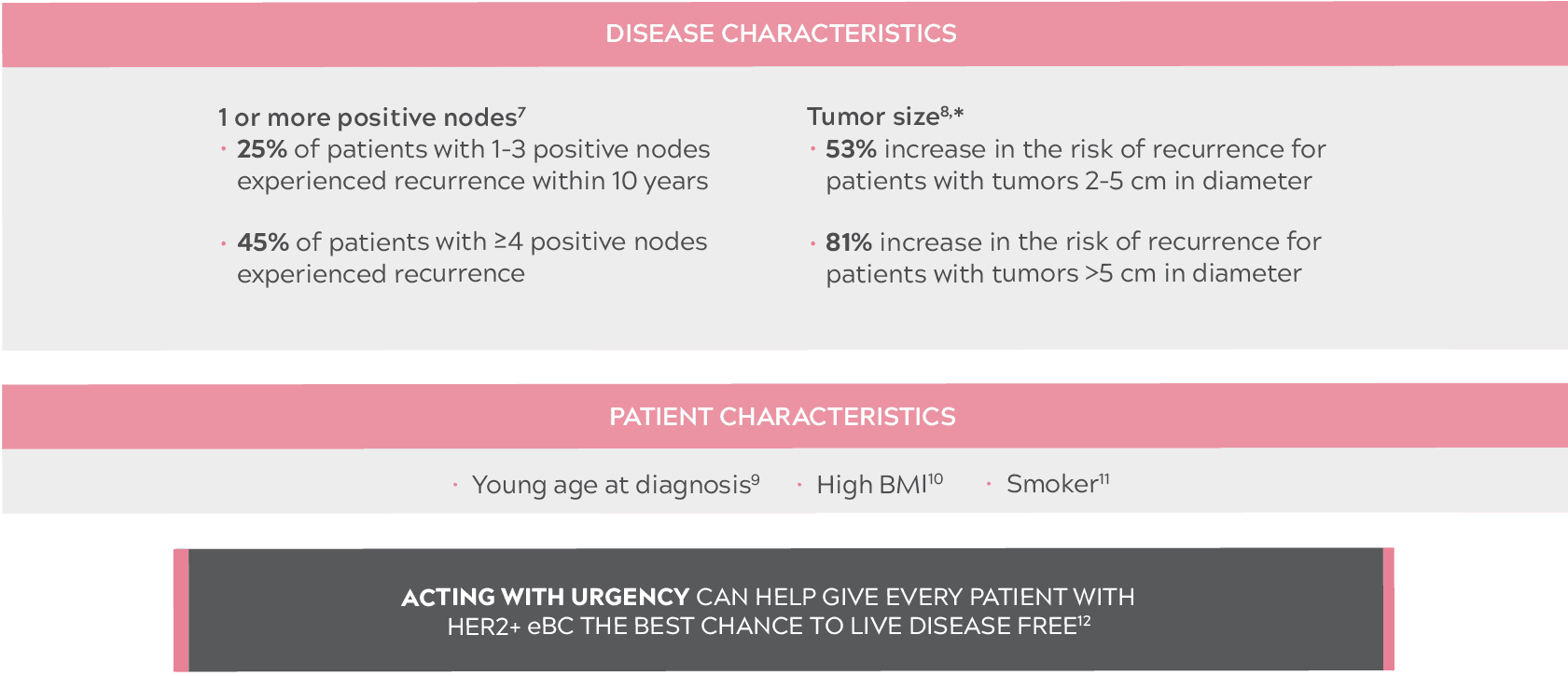
- Compared with tumors less than 2 cm in diameter.8
- BMI: body mass index; eBC: early-stage breast cancer; HER2+: human epidermal growth factor receptor 2–positive.
References:
- Loibl S, Mano MS, Untch M, et al. Phase III study of adjuvant ado-trastuzumab emtansine vs trastuzumab for residual invasive HER2-positive early breast cancer after neoadjuvant chemotherapy and HER2-targeted therapy: KATHERINE final IDFS and updated OS analysis. Presented at: 2023 San Antonio Breast Cancer Symposium; December 5-9, 2023; San Antonio, TX. GS03-12. Accessed February 29, 2024. https://medically.gene.com/global/en/unrestricted/oncology/SABCS-2023/sabcs-2023-presentation-loibl-phase-iii-study-of-adjuva.html
- von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628. doi:10.1056/NEJMoa1814017
- Untch M, Geyer CE Jr, Huang C-S, et al. Peripheral neuropathy, thrombocytopaenia, and central nervous system recurrence: an update of the phase III KATHERINE trial of post-neoadjuvant trastuzumab emtansine (T-DM1) or trastuzumab in patients with residual invasive HER2-positive breast cancer. Presented at: European Society for Medical Oncology (ESMO) Annual Meeting; September 27-October 1, 2019; Barcelona, Spain.
- Gianni L, Pienkowski T, Im Y-H, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791-800. doi:10.1016/S1470-2045(16)00163-7
- Leone JP, Leone BA. Breast cancer brain metastases: the last frontier. Exp Hematol Oncol. 2015;4:33. doi:10.1186/s40164-015-0028-8
- Piccart M, Procter M, Fumagalli D, et al; APHINITY Steering Committee and Investigators. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J Clin Oncol. 2021;39(13):1448-1457. doi:10.1200/JCO.20.01204
- Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195-1205. doi:10.1016/S0140-6736(16)32616-2
- Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2–positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744-3752. doi:10.1200/JCO.2014.55.5730
- Recurrent breast cancer. Mayo Clinic. Updated July 2, 2022. Accessed May 11, 2023. https://www.mayoclinic.org/diseases-conditions/recurrent-breast-cancer/symptoms-causes/syc-20377135
- Sun L, Zhu Y, Qian Q, Tang L. Body mass index and prognosis of breast cancer: an analysis by menstruation status when breast cancer diagnosis. Medicine (Baltimore). 2018;97(26):e11220. doi:10.1097/MD.0000000000011220
- Pierce JP, Patterson RE, Senger CM, et al. Lifetime cigarette smoking and breast cancer prognosis in the After Breast Cancer Pooling Project. J Natl Cancer Inst. 2014;106(1):djt359. doi:10.1093/jnci/djt359
- Chan A, Moy B, Mansi J, et al. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the phase III ExteNET trial. Clin Breast Cancer. 2021;21(1):80-91.e7. doi:10.1016/j.clbc.2020.09.014
Important Safety Information and Indications
Contraindications: None
Warnings and Precautions:
- Diarrhea: Manage diarrhea through either Nerlynx dose escalation or loperamide prophylaxis. If diarrhea occurs despite recommended prophylaxis, treat with additional antidiarrheals, fluids, and electrolytes as clinically indicated. Withhold Nerlynx in patients experiencing severe and/or persistent diarrhea. Permanently discontinue Nerlynx in patients experiencing Grade 4 diarrhea or Grade ≥2 diarrhea that occurs after maximal dose reduction.
- Hepatotoxicity: Monitor liver function tests monthly for the first 3 months of treatment, then every 3 months while on treatment and as clinically indicated. Withhold Nerlynx in patients experiencing Grade 3 liver abnormalities and permanently discontinue Nerlynx in patients experiencing Grade 4 liver abnormalities.
- Embryo-Fetal Toxicity: Nerlynx can cause fetal harm. Advise patients of potential risk to a fetus and to use effective contraception.
Adverse Reactions: The most common adverse reactions (reported in ≥5% of patients) were:
- Nerlynx as a single agent: diarrhea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, AST or ALT increased, nail disorder, dry skin, abdominal distention, epistaxis, weight decreased, and urinary tract infection.
- Nerlynx in combination with capecitabine: diarrhea, nausea, vomiting, decreased appetite, constipation, fatigue/asthenia, weight decreased, dizziness, back pain, arthralgia, urinary tract infection, upper respiratory tract infection, abdominal distention, renal impairment, and muscle spasms.
To report suspected adverse reactions, contact Puma Biotechnology, Inc. at 1-844-Nerlynx (1-844-637-5969) or FDA at 1-800-332-1088 or www.fda.gov/medwatch.
Drug Interactions:
- Gastric acid reducing agents: Avoid concomitant use with proton pump inhibitors. Separate Nerlynx by at least 2 hours before or 10 hours after H2-receptor antagonists. Or separate Nerlynx by at least 3 hours after antacids.
- Strong CYP3A4 inhibitors: Avoid concomitant use.
- P-gp and moderate CYP3A4 dual inhibitors: Avoid concomitant use.
- Strong or moderate CYP3A4 inducers: Avoid concomitant use.
- Certain P-gp substrates: Monitor for adverse reactions of P-gp substrates for which minimal concentration change may lead to serious adverse reactions when used concomitantly with Nerlynx.
Use In Specific Populations:
- Lactation: Advise women not to breastfeed.
Please see Full Prescribing Information.
Indications: Nerlynx® (neratinib) tablets, for oral use, is a kinase inhibitor indicated:
- As a single agent, for the extended adjuvant treatment of adult patients with early-stage HER2-positive breast cancer, to follow adjuvant trastuzumab-based therapy.
- In combination with capecitabine, for the treatment of adult patients with advanced or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 based regimens in the metastatic setting.