Help protect against progression in HER2+ metastatic breast cancer1,*
* After ≥2 prior anti-HER2—based regimens in the metastatic setting.
Nerlynx® + capecitabine may help protect against progression1,2
PFS in patients with HER2+ mBC vs lapatinib + CAPECITABINE1,2
29% PFS rate at 12 months vs 15% with lapatinib + capecitabine1
- Nerlynx + capecitabine significantly improved median PFS vs lapatinib + capecitabine: 5.6 months with Nerlynx + capecitabine vs 5.5 months with lapatinib + capecitabine (HR=0.76; 95% CI: 0.63-0.93; P=0.0059)1
- There was no significant difference in OS between Nerlynx + capecitabine vs lapatinib + capecitabine: 21.0 months with Nerlynx + capecitabine vs 18.7 months with lapatinib + capecitabine (HR=0.88; 95% CI: 0.72-1.07; P=0.2086)1
- The total number of patients remaining in the study at 24 months was 11 (9 patients receiving NERLYNX + capecitabine, 2 patients receiving lapatinib + capecitabine).1
- CI: confidence interval; HR: hazard ratio; mBC: metastatic breast cancer; OS: overall survival; PFS: progression-free survival.
Nerlynx safety in NALA1
Most common adverse reactions (≥5%)
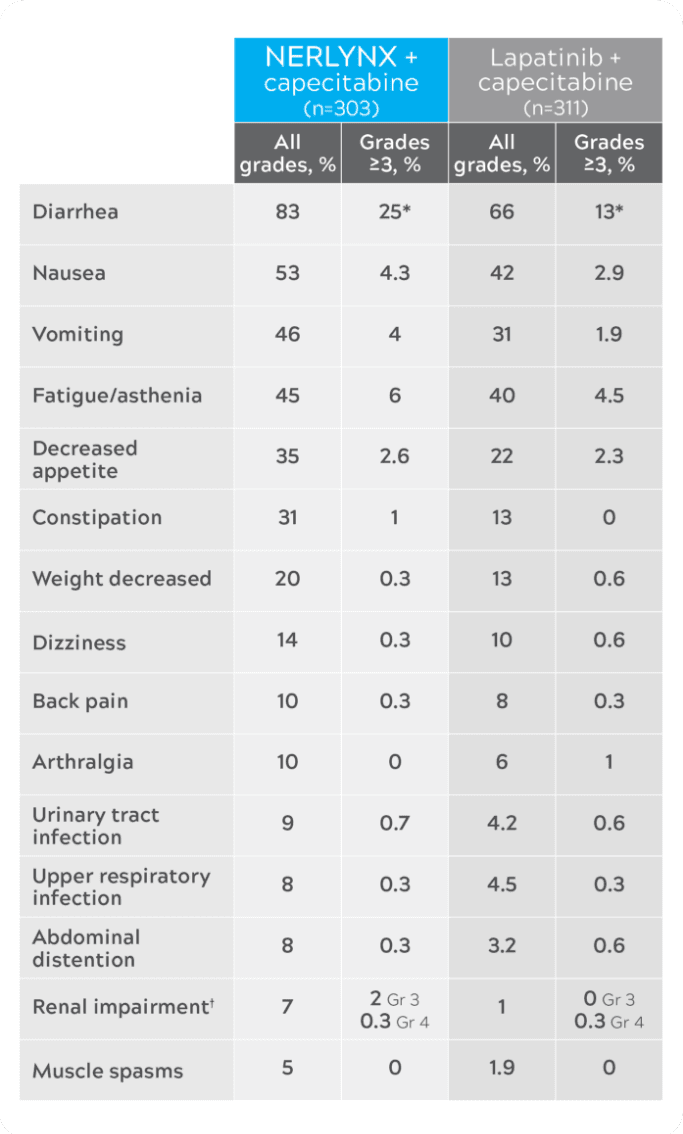
The USPI warnings and precautions do not include cardiac, pulmonary, or hematologic toxicities, or increased risk for secondary malignancy.1
- Discontinuation of Nerlynx or lapatinib due to any adverse event occurred in 10.9% of patients treated with Nerlynx + capecitabine vs 14.5% of patients treated with lapatinib + capecitabine2
Drug Interactions1
- Gastric acid–reducing agents: Avoid concomitant use with proton pump inhibitors. Separate Nerlynx by at least 2 hours before or 10 hours after H2-receptor antagonists. Or separate Nerlynx by at least 3 hours after antacids
- Strong CYP3A4 inhibitors: Avoid concomitant use
- P-gp and moderate CYP3A4 dual inhibitors: Avoid concomitant use
- Strong or moderate CYP3A4 inducers: Avoid concomitant use
- Certain P-gp substrates: Monitor for adverse reactions of P-gp substrates for which minimal concentration change may lead to serious adverse reactions when used concomitantly with Nerlynx
- No grade 4 diarrhea.1
- Renal impairment includes acute kidney injury, blood creatinine increased, renal failure, and renal impairment.1
- CYP3A4: cytochrome P450 3A; P-gp: P-glycoprotein.
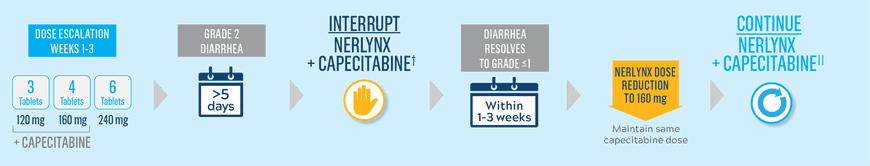
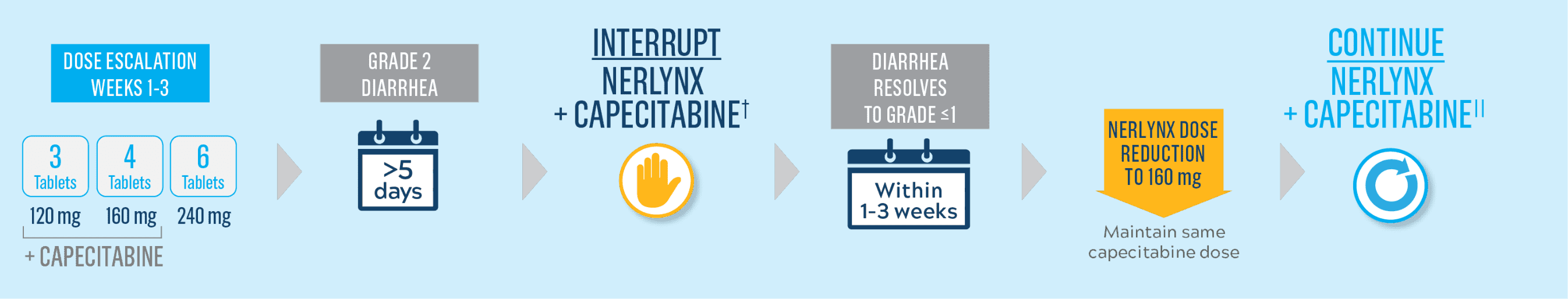
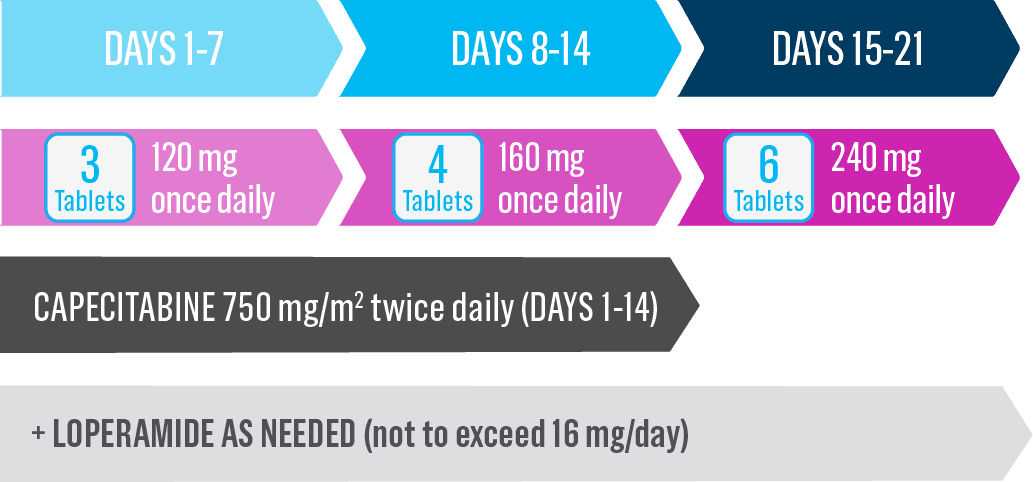
Start Nerlynx at a lower dose and titrate up to the full recommended dose to help manage diarrhea1
Cycle 1—Nerlynx dose escalation1

Cycle 2 and beyond—full recommended Nerlynx dose1
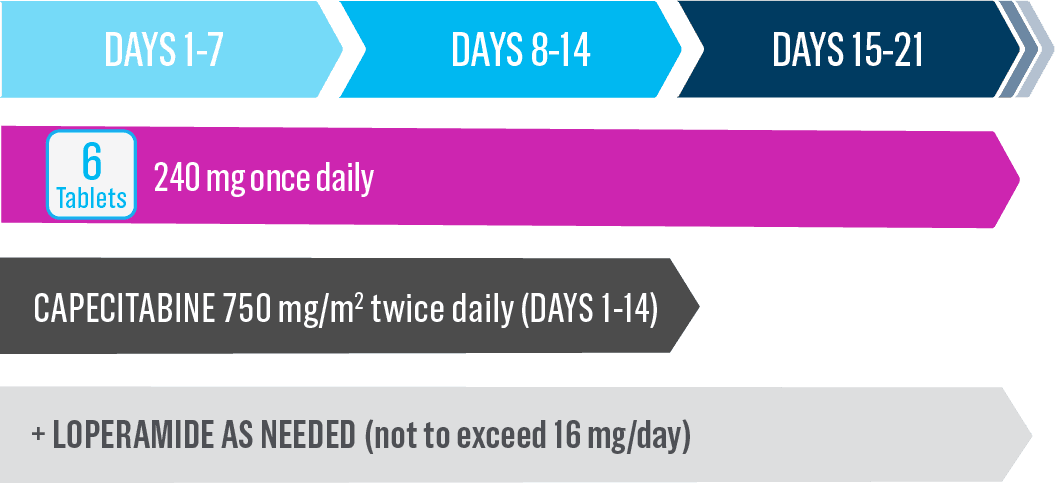

- Nerlynx is an oral once-daily therapy taken continuously until disease progression or unacceptable toxicities1
- Capecitabine is taken with water within 30 minutes after a meal on days 1 to 14 of each 21-day cycle3,*
- If diarrhea occurs, treat with antidiarrheal medications, fluids, and electrolytes as clinically indicated1
- Refer to the capecitabine Prescribing Information when NERLYNX is used in combination with capecitabine.
- mBC: metastatic breast cancer.
Support for patients prescribed NERLYNX*
- Access and coverage
- Ongoing patient support
Puma Patient Lynx programs are subject to change or to be discontinued without notice. Limitations apply and certain programs are subject to eligibility criteria. For full terms and conditions, call 1-855-816-5421.
Download the Nerlynx Dosing and Administration Guide for additional information to help your patients get started with Nerlynx
Once-daily oral dosing with Nerlynx
IMPORTANT DOSING INFORMATION1
- The recommended dose is 240 mg once daily*
- A 2-week dose escalation for Nerlynx may also be initiated
- Instruct patients to take Nerlynx with food at approximately the same time every day
- Nerlynx tablets should be swallowed whole (not be chewed, crushed, or split prior to swallowing)
- Patients with metastatic HER2+ breast cancer should take Nerlynx until disease progression or unacceptable toxicities
- If a patient misses a dose, do not replace a missed dose, and instruct the patient to resume Nerlynx with the next scheduled daily dose
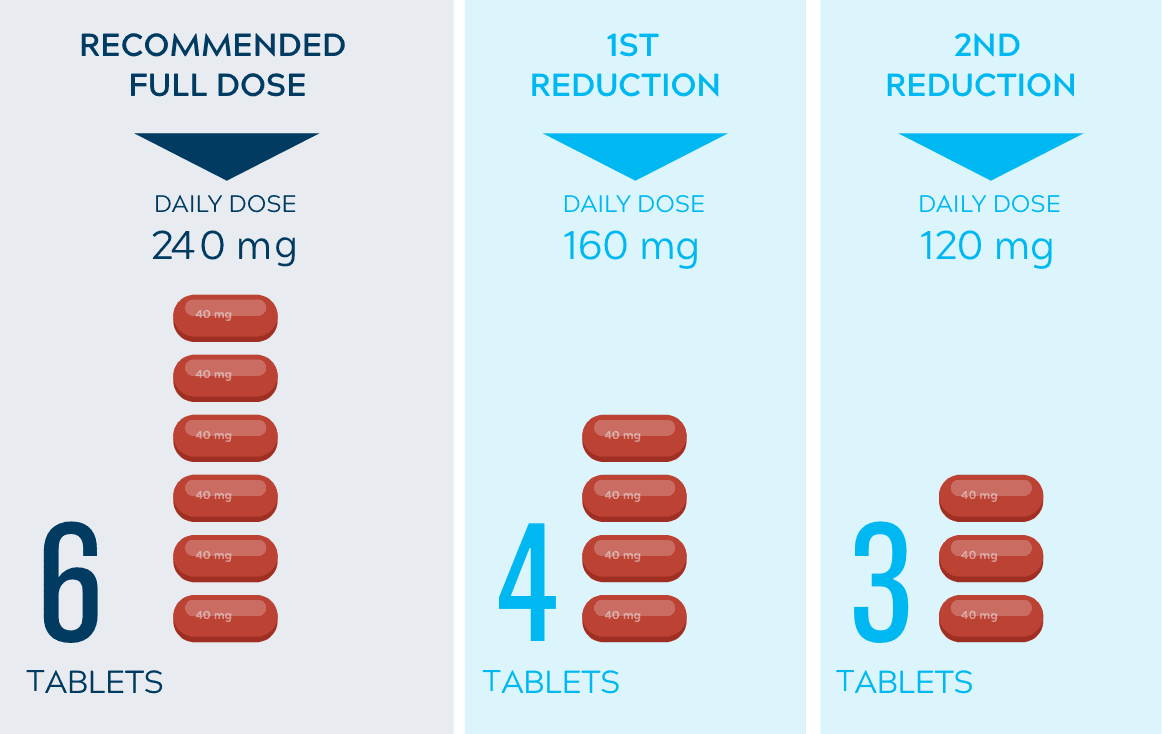
- Dose interruptions and/or dose reductions are recommended based on individual safety and tolerability
- Hepatic impairment: reduce the starting dose to 80 mg in patients with severe hepatic impairment
- Discontinue Nerlynx for patients with adverse reactions that fail to recover to grade 0-1 or baseline, with toxicities that result in a treatment delay >3 weeks, or if unable to tolerate 120 mg daily
- If diarrhea occurs, treat with antidiarrheal medications, fluids, and electrolytes as clinically indicated.1
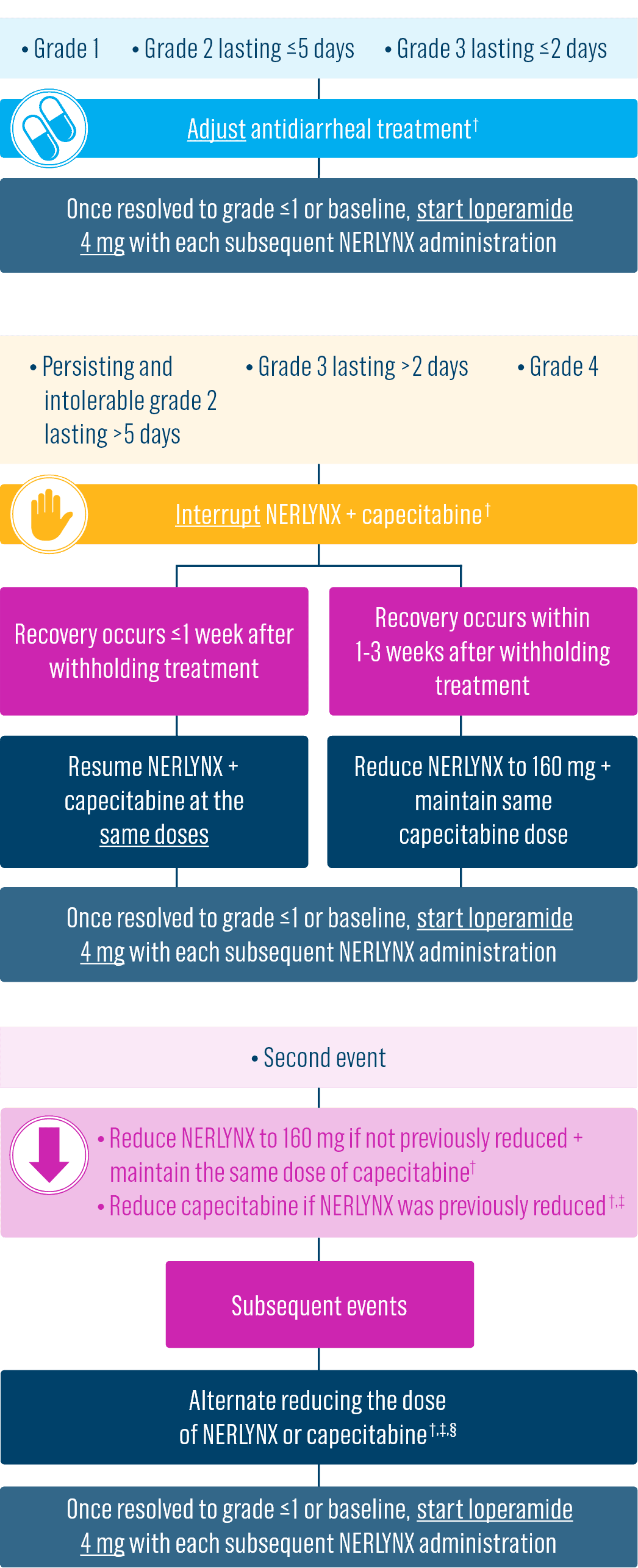
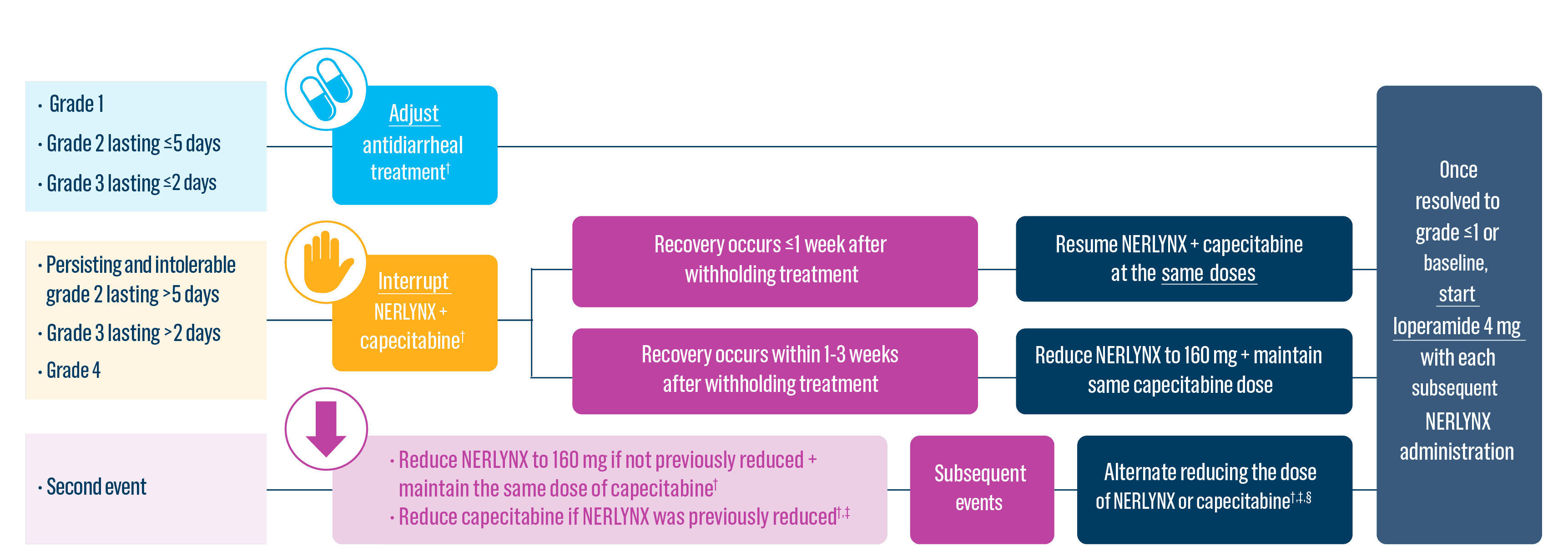
Dose adjustments to help manage side effects1


© 2017 Puma Biotechnology, Inc.
References:
- Nerlynx [package insert]. Los Angeles, CA: Puma Biotechnology, Inc.
- Saura C, Oliveira M, Feng Y-H, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138-3149. doi:10.1200/JCO.20.00147
- Puma Biotechnology, Inc. Data on file.
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. November 27, 2017. Accessed October 29, 2025. https://dctd.cancer.gov/research/ctep-trials/trial-development
Important Safety Information
Warnings and Precautions:
- Diarrhea: Manage diarrhea through either Nerlynx dose escalation or loperamide prophylaxis. If diarrhea occurs despite recommended prophylaxis, treat with additional antidiarrheals, fluids, and electrolytes as clinically indicated. Withhold Nerlynx in patients experiencing severe and/or persistent diarrhea. Permanently discontinue Nerlynx in patients experiencing Grade 4 diarrhea or Grade ≥2 diarrhea that occurs after maximal dose reduction.
Indications
Nerlynx® (neratinib) tablets, for oral use, is a kinase inhibitor indicated:
- As a single agent, for the extended adjuvant treatment of adult patients with early-stage HER2-positive breast cancer, to follow adjuvant trastuzumab-based therapy.
Important Safety Information
Warnings and Precautions:
Warnings and Precautions:
- Hepatotoxicity: Monitor liver function tests monthly for the first 3 months of treatment, then every 3 months while on treatment and as clinically indicated. Withhold Nerlynx in patients experiencing Grade 3 liver abnormalities and permanently discontinue Nerlynx in patients experiencing Grade 4 liver abnormalities.
- Embryo-Fetal Toxicity: Nerlynx can cause fetal harm. Advise patients of potential risk to a fetus and to use effective contraception.
Adverse Reactions: The most common adverse reactions (reported in ≥5% of patients) were:
- Nerlynx as a single agent: diarrhea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, AST or ALT increased, nail disorder, dry skin, abdominal distention, epistaxis, weight decreased, and urinary tract infection.
- Nerlynx in combination with capecitabine: diarrhea, nausea, vomiting, decreased appetite, constipation, fatigue/asthenia, weight decreased, dizziness, back pain, arthralgia, urinary tract infection, upper respiratory tract infection, abdominal distention, renal impairment, and muscle spasms.
To report suspected adverse reactions, contact Puma Biotechnology, Inc. at 1-844-Nerlynx (1-844-637-5969) or FDA at 1-800-332-1088 or www.fda.gov/medwatch.
Drug Interactions:
- Gastric acid reducing agents: Avoid concomitant use with proton pump inhibitors. Separate Nerlynx by at least 2 hours before or 10 hours after H2-receptor antagonists. Or separate Nerlynx by at least 3 hours after antacids.
- Strong CYP3A4 inhibitors: Avoid concomitant use.
- P-gp and moderate CYP3A4 dual inhibitors: Avoid concomitant use.
- Strong or moderate CYP3A4 inducers: Avoid concomitant use.
- Certain P-gp substrates: Monitor for adverse reactions of P-gp substrates for which minimal concentration change may lead to serious adverse reactions when used concomitantly with Nerlynx.
Use In Specific Populations:
- Lactation: Advise women not to breastfeed.
Please see Full Prescribing Information.
Indications
Nerlynx® (neratinib) tablets, for oral use, is a kinase inhibitor indicated:
- In combination with capecitabine, for the treatment of adult patients with advanced or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 based regimens in the metastatic setting.